For perfect gases the coefficient of thermal growth is 1/273 at 0 levels C. A temperature increase of 1 degrees C alters the volume of a gas by 0.366 %. At 25 degrees C the volume of 1 g of water is 1.00296 mL, at 26 degrees C the volume is 1.00323 or a modification of about 0.026 %. London dispersion pressures occur between atoms or molecules of nonpolar substances. Monoatomic atoms, diatomic particles and nonpolar compounds (CH4, CCl4, BF3, BeH2, and so on) are all defined by a symmetric sharing of electrons in the atom or particle.
Oxygen intercalates gradually right into solid fullerites saved in air however can typically be removed by pumping at raised temperature level. However, intercalated oxygen can additionally respond to develop fullerene oxide.
Compounds That Exist As Gases.
Both cyclohexene as well as cyclobutane are essentially nonpolar particles, yet cyclobutane has a substantially lower molecular biofeedback mass than cyclohexene, which again has greater than four carbon atoms. We therefore forecast that cyclobutane is more than likely a gas at area temperature level as well as stress, while cyclohexene is a fluid. Actually, with a boiling point of only 12 ° C, contrasted to 83 ° C for cyclohexene, cyclobutane is indeed a gas at room temperature as well as stress.
Who discovered Monatomic gold?
David Hudson went public with his findings in the early 1990s. He spent millions of dollars and dedicated the next decade of his life to figuring out how to obtain concentrated Ormus and work with his new discovery.
3. Known Ormus (m-state) Elements.ElementAtomic NumberGold (Au)79Mercury (Hg)8010 more rows•Feb 7, 2018
For instance, carbon as well as oxygen can combine to develop CARBON DIOXIDE or CO; sulfur as well as oxygen can integrate to create SO2 or SO3. To identify these substances from each other, Greek prefixes are made use of to mark the varieties of atoms of one or both aspects in the molecule. Consequently, CARBON DIOXIDE is called co2 as well as Carbon Monoxide is called carbon monoxide; SO2 is sulfur dioxide as well as SO3 is sulfur trioxide.
Molar Mass And Also % Composition.
After that, call the much more electronegative aspect as if the a lot more electronegative component is a simple anion (ending with-- ide). Exactly how does one know which component is the electropositive element? If one follows this rule, then, SO2 would certainly be called sulfur oxide, as well as CO would be called carbon oxide. Really often, 2 nonmetals can combine to form greater than one compound.
- 3B, in which it can be seen that the dynamics of the monatomic ions in the liquid phase are spatially and temporally heterogeneous in their variation distances, instructions, and times.
- Much of the elements and compounds we have come across so far are usually found as gases; a few of the more common ones are detailed in Table 10.2 "Some Common Compounds That Are Gases at 25 ° C and also 1.0 atm".
- As an instance, we take the trajectory of ion C in Fig.
- 3C. The numbers in the figure correspond to the atomic placements in the corresponding frameworks.
- Additionally, most of the straightforward covalent oxides of the nonmetals are gases, such as Carbon Monoxide, CO2, NO, NO2, SO2, SO3, as well as ClO2.
- Ultimately, most of the frequently made use of cooling agents, such as the chlorofluorocarbons as well as the hydrochlorofluorocarbons, which were discussed in Chapter 3 "Chemical Reactions", are gases.
Atoms of these components are extremely steady due to their stable digital setup. Therefore, they do not require to undergo any type of chain reactions in order to get supported. These worthy gases consist of Helium, Neon, Argon, Krypton, Xenon and Radon. The term monatomic is utilized to call compounds composed of bits consisting of single atoms. Simply put, the atoms of monatomic compounds are not bound per other. This is because these elements are secure as solitary atoms.
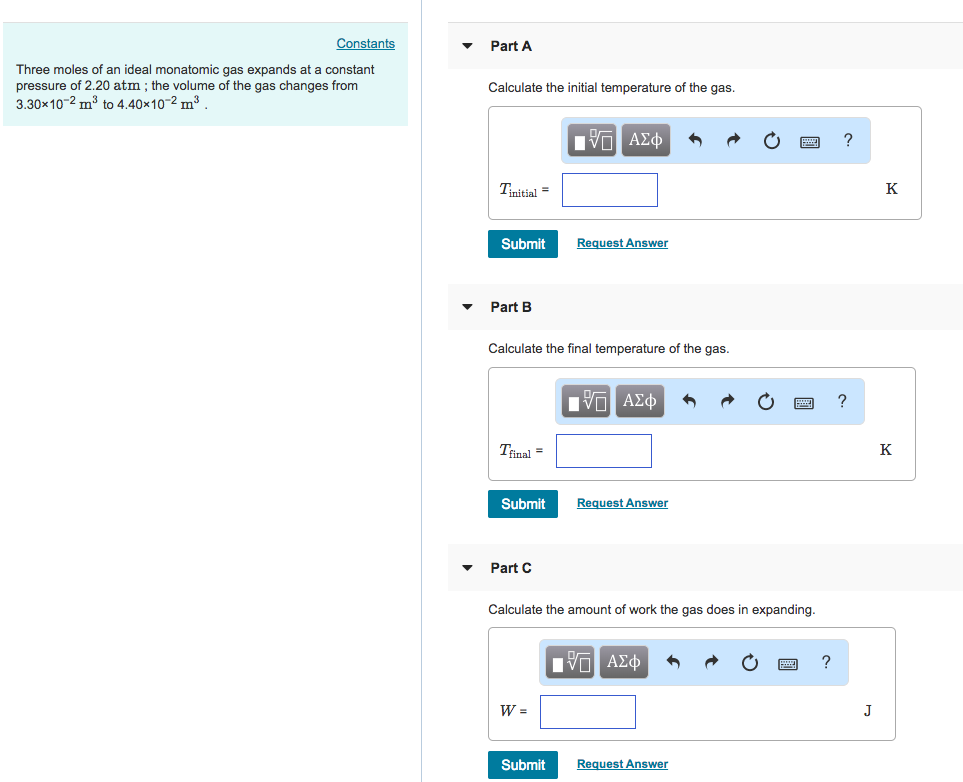
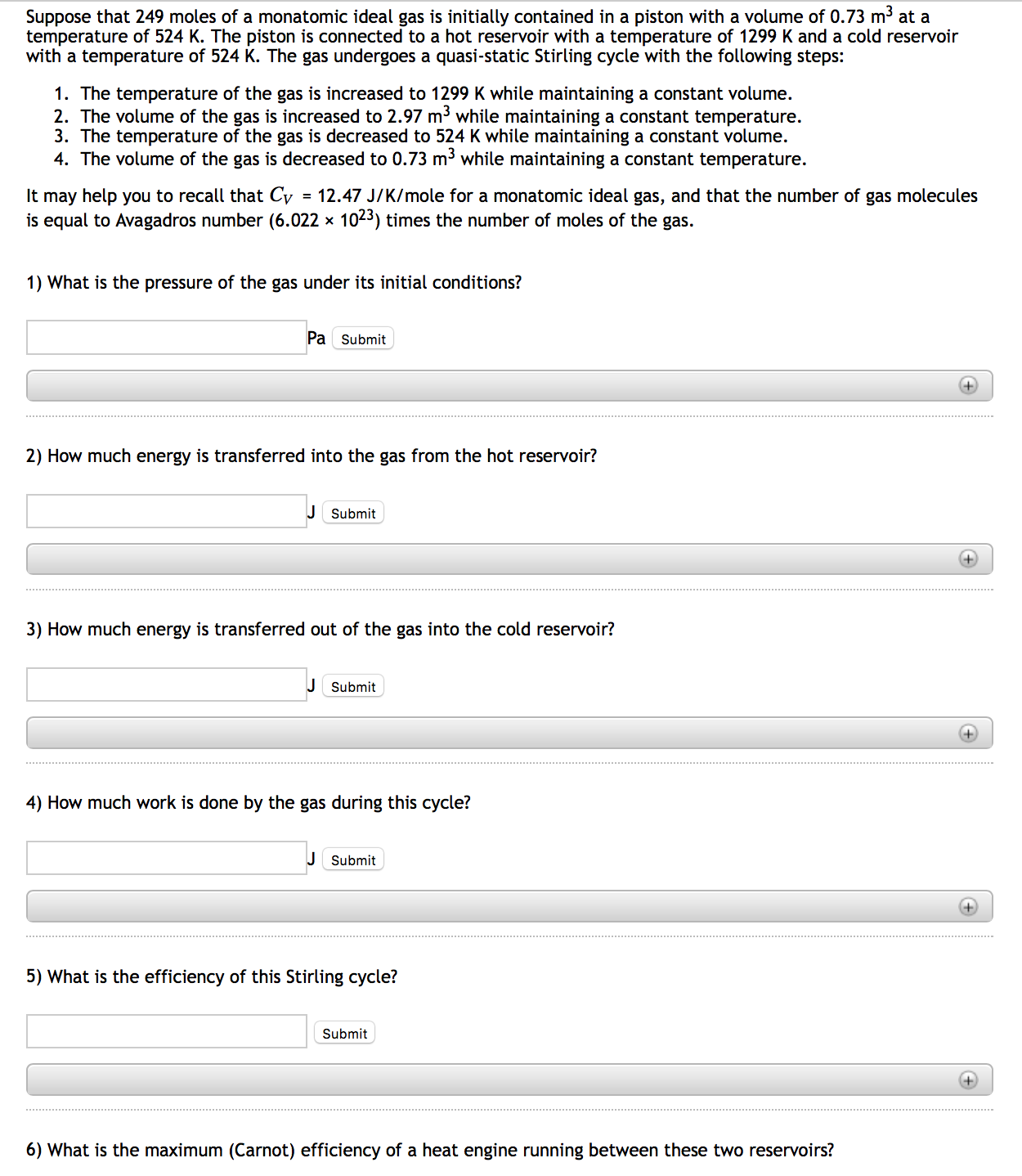
During an adiabatic process, the temperature level of 3.92 moles of a monatomic optimal gas decreases from 485 ° C to 205 ° C. For this gas, locate the job it does, the heat it exchanges with its surroundings, as well as the change in its internal energy. IP If 8.00 moles of a monatomic excellent gas at a temperature of 245 K are expanded isothennally from a volume of 1.12 L to a volume of 4.33 T, determine the job done as well as the warm circulation into or out of the gas. If the variety of moles is doubled, by what aspects do your solution to components as well as alter?
Relevant Questions.
The most effective examples of monatomic substances are inert gases. The noble gases are also gases at STP, yet they are monatomic. The homonuclear diatomic gases as well as worthy gases with each other are called "important gases" or "molecular gases", to differentiate them from various other gases that are chemical substances. For example; Nobel gases do not develop molecules with other atoms as they have octet setup such as Ne, Xe, Registered nurse etc . Only Helium is an outstanding situation in which 1s2 setup is secure one.